CITI Training Guide
Purpose
This guide has been drafted by Saint Francis Hospital's Research Development Office to use as a reference to complete the Collaborative Institutional Training Initiative (CITI) training as required by the Trinity Health Of New England IRB.
IRB Policy
Trinity Health Of New England IRB requires all Investigators, study coordinators, and research staff to complete training in the protection of human subjects prior to receiving IRB approval of a protocol. The purpose of this training is to ensure that investigators conducting research are qualified to conduct the study, to uphold Belmont Report principles, and ensure compliance with the regulations and the requirements of the IRB in the approved protocol. Minimum standards for approved training programs require the inclusion of the principles of the Belmont Report. Currently, the IRB provides and accepts CITI training. Personnel can complete training at www.citiprogram.org. All study staff are required to complete: Human Subject Research Training; (Biomedical Research OR Social Behavioral Research) Responsible Conduct of Research, Good Clinical Practice-Biomedical Research (GCP) and Conflicts of Interest (COI). A quiz is taken after each of the modules, and upon completion of the training a course completion record is issued by the Collaborative Institutional Training Initiative (CITI) group.
Off-site investigators collaborating with an investigator from an approved site must create or affiliate their CITI training account with Trinity Health Of New England (Hartford, CT) and complete all courses that the Trinity Health Of New England IRB requires of their staff. If the required training courses were taken at another institution and are still current, they will be carried over to the Trinity Health Of New England CITI account.
Training of medical students and house staff usually is required, but assessed on a case-by-case basis, depending on the type of research being conducted, and on whether the mentors are involved in the training process. The minimum training requirement for conducting record review studies is the successful completion of the CITI on-line training modules in “Data Specimens and Record Review” and the Trinity Health Of New England required HIPAA
training for all employees.
Subsequent to the initial training, the IRB requires all investigators, study coordinators, and research staff to complete a CITI refresher course via www.citiprogram.org. The refresher course is to be completed every 3 years.
Individuals who do not meet the training requirements of the IRB may not be involved in human research activities. The IRB will take appropriate action to withhold or reverse the approval of research when training requirements are not met. The IRB will not approve protocols until training requirements have been fulfilled. When an investigator, study coordinator, or research assistant is added to a protocol after the initial approval, training requirements must be met before the individual is involved in human research activities.
Trinity Health Of New England IRB Policy
Steps and Procedures
- Navigate to the CITI website: https://www.citiprogram.org/
- For a new CITI Account
- Click the Register button on the main page of the CITI website and enter the appropriate information as indicated for the following:
- Organization Affiliation: Trinity Health – Trinity Health Of New England (Hartford, CT)
- (type: Trinity Health Of…. and it should appear)
- Personal Information: Enter name & email address
- Create Username & Password
- Demographic Information
- Option for receiving Continuing Education Unit (CEU) (Charges may apply)
- Institutional Contact Information – (Enter the appropriate Trinity Health Of New England Hospital)
- If you already have a CITI account
- Log in to CITI and confirm you are affiliated with Trinity Health – Trinity Health Of New England (Hartford, CT).
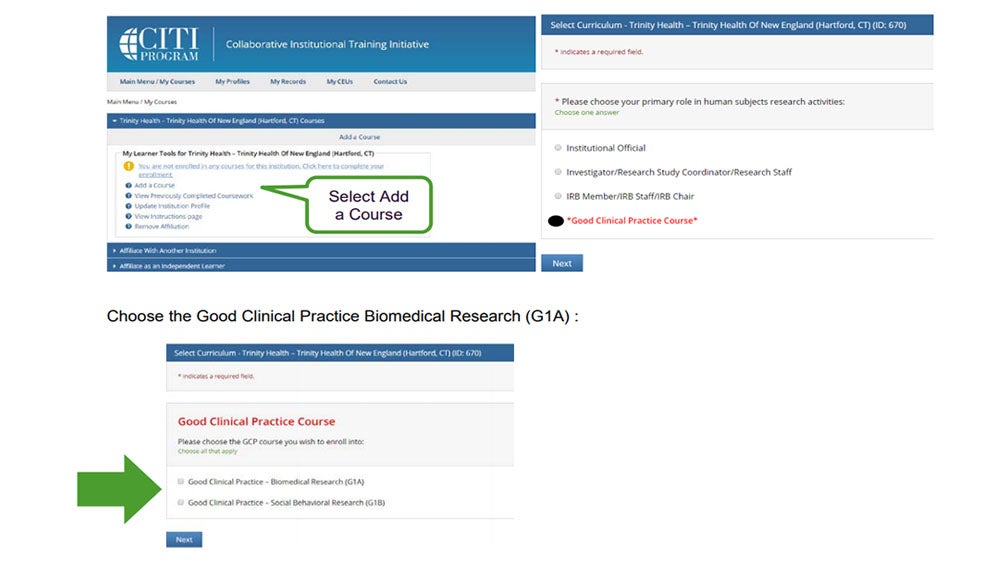
•Select Add a Course from the menu
•Then choose *Good Clinical Practice Course*
- Log in to CITI and confirm you are affiliated with Trinity Health – Trinity Health Of New England (Hartford, CT).
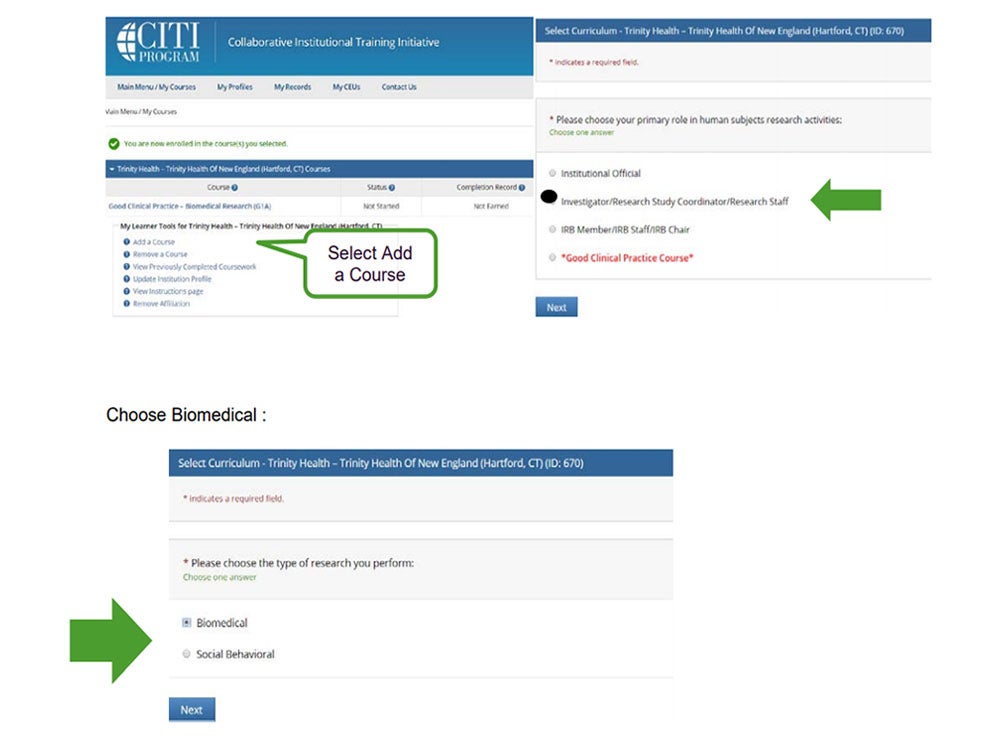
- Return to Add a Course and select Investigator/Research Study Coordinator/Research Staff as indicated
below:
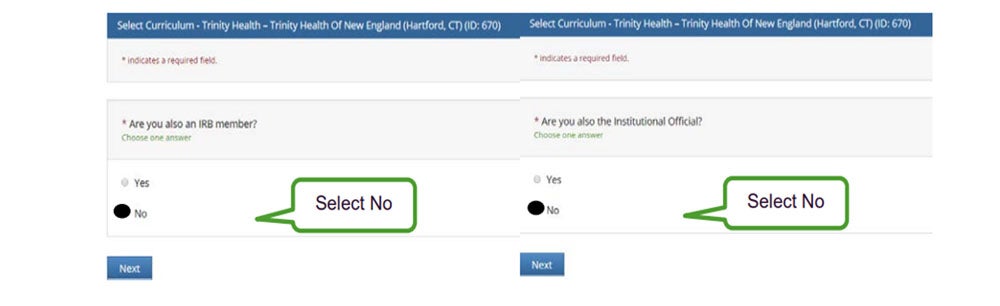
- Answer each of the questions below as indicated:
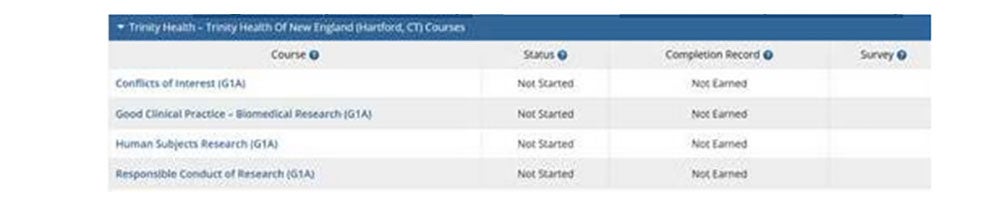
- Confirm you have selected/registered for all of the coursed listed below (As required by the Trinity Health Of New England IRB):
- Conflicts of Interest (G1A)
- Good Clinical Practice - Biomedical Research (G1A)
- Human Subjects Research (G1A)
- Responsible Conduct of Research (G1A)
Only complete Required Modules: Plagiarism (RCR-Basic) & Research Misconduct (RCR-Basic)
Forgot your CITI Username and Password?
- To retrieve your Username or Password click Forgot from the CITI login page.
- To retrieve your CITI Username: enter your email address.
- To retrieve your CITI Password: enter your email address and Username.
